DESIGN FMEA – MINIMISING RISK
We ensure risk is minimised through the design process by undertaking Design Failure Mode Effects Analysis, as part of the development process.

MEDICAL DEVICE STANDARDS
We ensure your products meet the required standards. We have associate regulatory specialists to undertake this for any medical device development.
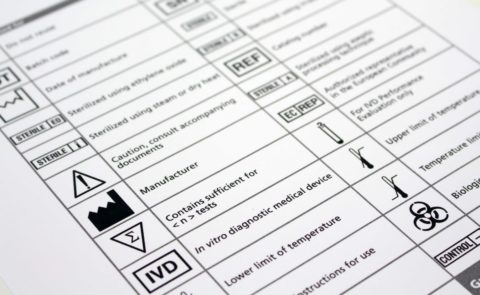
MEDICAL DEVICE SYMBOLS
We ensure the relevant symbols are displayed on your medical device. These will either be engraved on the plastic moulding or on a printed label.

REGULATORY PACKAGING LABEL
We can design your product label, ensuring the required information, such as medical device symbols and expiry dates are clearly shown
"We ensure your product meets the required standards"
Gm Design Development UK ensures the regulatory requirements for your medical device are achieved
We understand it is crucial to understand the regulatory requirements when developing healthcare and medical products. We have over 15 years knowledge of medical standards, and know how critical it is to fully understand them at the start of any development project. It ensures your product will meet all the requirements, as well as saving considerable re-development costs.
Regulatory Associate
We work closely with Meddiquest Limited, a specialist regulatory affairs consultancy, ensure all your regulatory requirements are met when developing products.
MEDDIQUEST LIMITED - Regulatory Affairs Consultancy
Meddiquest services the Medical, Scientific and BioTech sectors, and has in-depth knowledge of the medical regulatory requirements for US, European and other markets.
DFMEA
We ensure risk is minimised through the design process by undertaking Design Failure Mode Effects Analysis, often organising the whole team to take part. Scores are calculated and rectifications made to improve the scoring to a satisfactory level.
Regulatory advice and testing
As part of the development process, we undertake early R&D and testing to prove key aspects of the design.
ISO standards
We ensure the design of your product or device meets the required standards, such as EN 60601 for electronic medical products. Ensuring the relevant standards are incorporated into the specification at the start of the development is critical.
510K/FDA
We can help with ensuring meets the requirements of 510K if your product is to be sold in the US. A regulatory specialist will assist you with the requirements and deliverables needed.