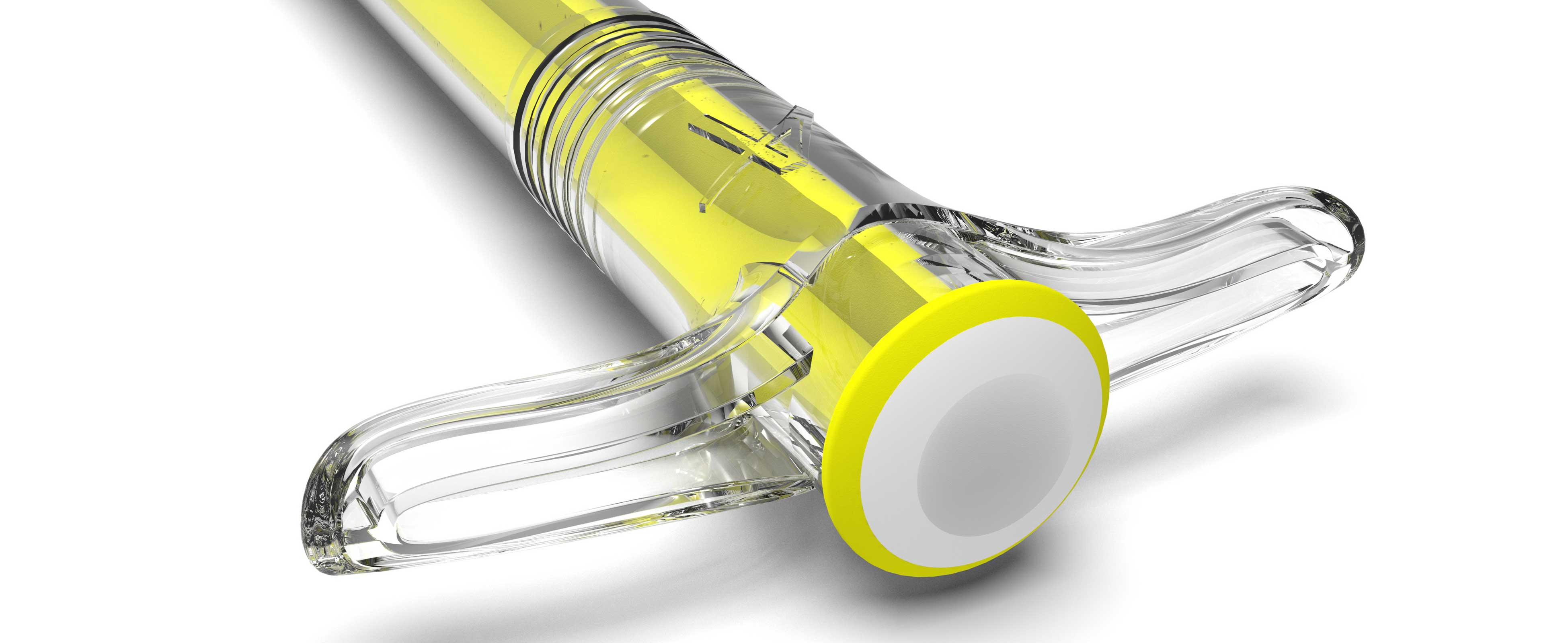


Apatech bone graft substitute dispenser
Apatech engaged Gm Design Development to develop a premium syringe to both house and dispense their osteoinductive bone graft substitute. One important specification was to design a syringe with an excellent moisture vapour transmission Rate (MVTR) to preserve the bone graft substitute. We researched medical grade resins and used a Cyclic olefin copolymer (COC) called Topas to achieve this.
The end cap and elastomer seals were produced in a medical grade resin and the combination of these along with the Topas moulded syringe body delivered the effective barrier required.
The crystal clear and rigid syringe body gave the device the premium feel required, whilst at the same time maintaining a simple-to-mould syringe body with an ergonomic grip.
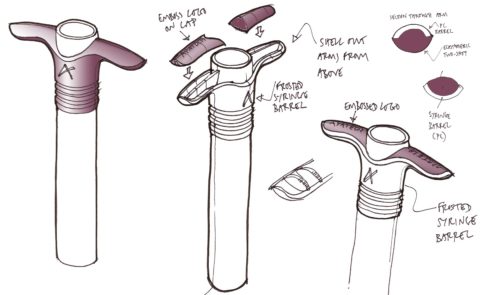
BONE GRAFT SYRINGE CONCEPTS
We developed a range of concept designs for the syringe, exploring grip options and how best to deliver the premium feel required.

BONE GRAFT SYRINGE PROTOTYPES
Prototypes were produced to evaluate the syringe designs, testing the ergonomics of the two different sizes of syringes
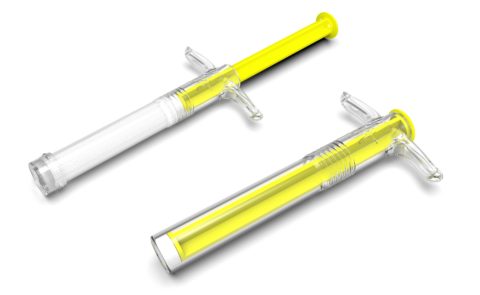
BONE GRAFT SYRINGE DESIGNS
Two sizes of syringes were designed. Moulded in Topas for a premium appearance and excellent MVTR properties, with end caps completing the seal.
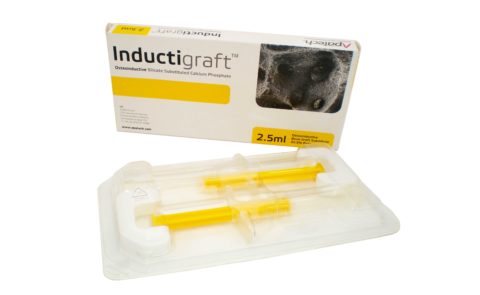
BONE GRAFT STERILE PACKAGING
The syringes filled with osteoinductive bone graft substitute are packaged in a vacuum formed sterile medical tray, housed in a protective cardboard box.