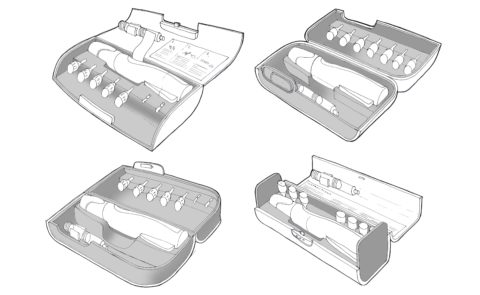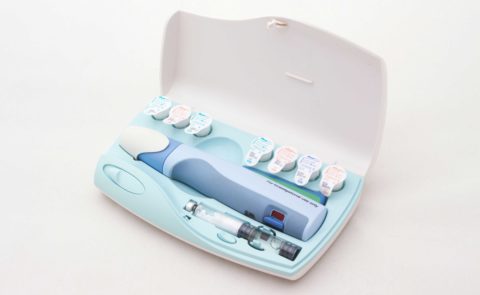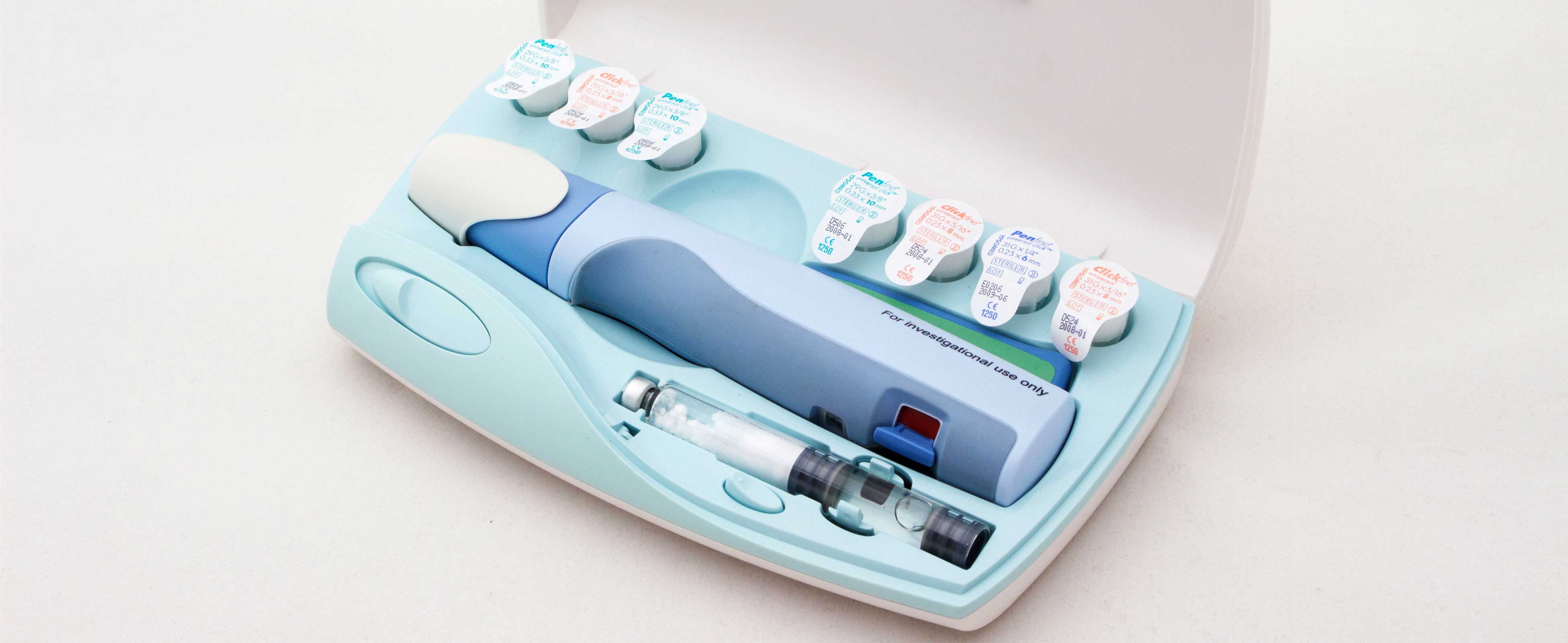


Nycomed Preobox compliance system
The high cost of a new self-injected osteoporosis pharmaceutical was the focus of a project for Nordic company Nycomed. (Now Takeda). The aim was to help encourage users to comply, over a lengthy course of self-administered treatment when no immediate improvement would be felt. An electronic compliance case was considered, but would be developed as a MkII product. This case was developed in-time for the launch of the new drug. (Developed whilst at Pearson Matthews).
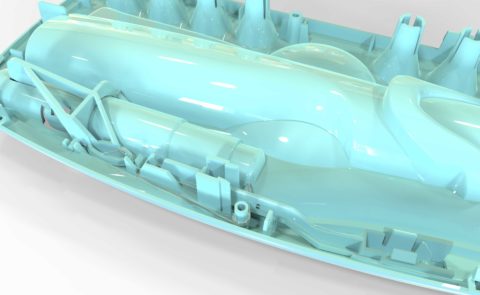
We finalised the design fully in 3D CAD and prepared a package of component and assembly drawings for the toolmaker. We worked with the toolmaker confirming all the details on the Tool GA.
We selected the Far East manufacturer for the product and managed the manufacturing stage of the project. The product was validated and delivered directly from the manufacturer to the customer.